Recent research(1)
Analysis of DNase II knockout mice
DNase II and DLAD (DNase II-like acid DNase) are acidic DNases. DNase II is expressed in almost all cells including macrophages. Intracellularly, DNase II is located in lysosomes and it plays a crucial role in degrading DNA of apoptotic cells engulfed by macrophages.
1) Analysis of DNase II knockout mice
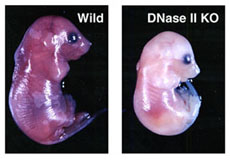
DNaseII is indispensable for the definitive erythropoiesis in mouse fetal liver. No live DNaseII-null mice were born, owing to severe anemia. DNaseII in macrophages appear to be responsible for destroying the nuclear DNA expelled from erythroid precursor cells.
2) Lethal anemia caused by IFN-beta
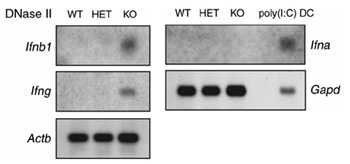
The livers of DNase II-deficient mouse embryos contain many macrophages carrying undigested DNA, and the embryos die in utero. Analysis of gene expression with a gene array showed that IFN-inducible genes were strongly activated in the livers of DNaseII-deficient embryos, and by in situ hybridization analysis, it was found that IFN-beta expression was induced in the resident liver macrophages. When the DNase II-deficient mice were crossed with mice deficient in type I IFN receptor, the mice were born healthy. These results indicate that the inability to degrade DNA derived from erythroid precursors results in interferon-beta production that induces expression of a specific set of IFN-responsive genes associated with embryonic lethality in DNase II-deficient mice.
3) Chronic polyarthritis caused by undigested mammalian DNA
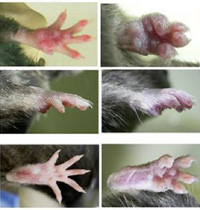
A large amount of chromosomal DNA is degraded during programmed cell death and definitive erythropoiesis. Macrophage rapidly engulf these cells or enucleated nuclei, and DNase II in lysosomes of macrophages digest the chromosomal DNA. To determine the physical role of DNase II, we established the mice with an induced deletion of DNase II gene (DNase IIΔ/-). Both DNase IIΔ/- mice and DNase II/type I IFN receptor double knockout mice, which are born normal, develop polyarthritis. Inflammatory cytokines were upregulated in affected joints of these mice, and administration of anti-TNF-α antibody prevented the developmenet of arthritis. These results indicate that a failure in degrading mammalian DNA can lead to the development of polyarthritis resembling human rheumatoid arthritis.
http://www.fbs.osaka-u.ac.jp/publications/nagata2006-1/
4) Phosphatidylserin-dependent engulfment of nuclei expelled from erythroid precursor cells
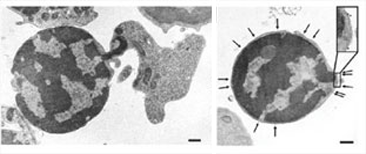
How do macrophages engulf nucleis expelled from erythroid precursor cells? In our body, 2 x 1011 red blood cells are generated everyday, suggesting that macrophages engulf as much as 2 x 1011nucleis everyday.
Here we show that the nuclei are engulfed by macrophages only after they are disconnected from reticulocytes, and that phosphatidylserine, which is often used as an 'eat me' signal for apoptotic cells, is also used for the engulfment of nuclei expelled from erythroblasts.
